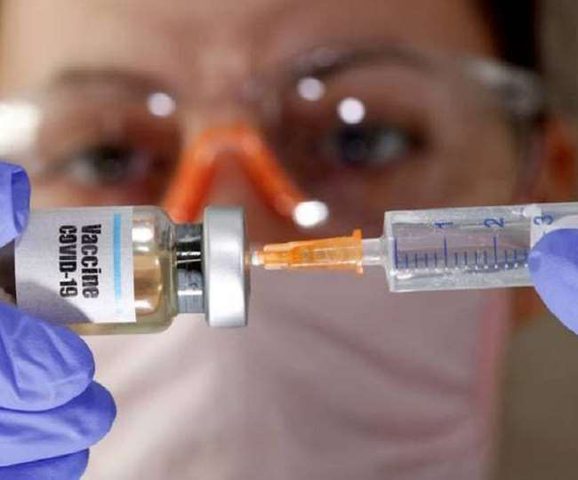
An-Najah News - The British official health authorities announced today, Wednesday, the approval of a vaccine developed by the University of "Oxford, in cooperation with the company," Astrasenica ", to prevent the virus (Corona emerging - Covid 19).
The Medical and Health Products Management Agency (MHRA) confirmed that the vaccine, whose efficacy has been proven by clinical trials of 90 percent, is a safe vaccine and will be used in Britain from the fourth of next January.
She explained that the government had previously requested 100 million doses of the vaccine, which differs from the vaccine (Vaser-Biontech) because it coughed up production and requires storage of regular refrigerators, adding that the vaccination with this vaccine will also be for the elderly and health sector employees before it is circulated.
For his part, British Health Minister Matt Hancock confirmed in a press statement that the (Oxford) vaccination campaign will start next Monday to give a major boost to the campaign that started three weeks ago.
He considered the presence of two vaccines in the hands of the British health authorities to expand and speed up the vaccination process as a key to returning life to its normal course.
It is noteworthy that the British authorities granted on the second day of this month the official approval of the vaccine (Vaser-Biontech), which experts say is 95 percent effective against the virus.