An-Najah News - A new vaccine that protects against Covid-19 is nearly 95% effective, early data from US company Moderna shows.
The results come hot on the heels of similar results from Pfizer, and add to growing confidence that vaccines can help end the pandemic.
Both companies used a highly innovative and experimental approach to designing their vaccines.
Moderna says it is a "great day" and they plan to apply for approval to use the vaccine in the next few weeks.
However, this is still early data and key questions remain unanswered.
Both vaccines use the same approach of injecting part of the virus's genetic code in order to provoke an immune response.
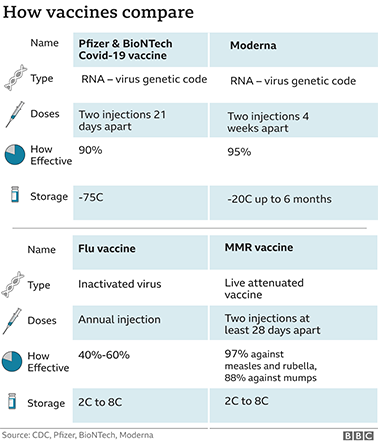
The preliminary data we have seen so far is very similar - around 90% protection for the Pfizer/BioNTech vaccine and around 95% for Moderna's.
A final analysis of the Phase 3 trial of Pfizer's coronavirus vaccine shows it was 95% effective in preventing infections, even in older adults, and caused no serious safety concerns, the company said Wednesday.
The company counted 170 cases of coronavirus infection among volunteers who took part in the trial. It said 162 infections were in people who got placebo, or plain saline shots, while eight cases were in participants who got the actual vaccine. That works out to an efficacy of 95%, Pfizer said.
The data show Pfizer's initial claim of a better than 90% efficacy -- a claim that stunned and pleased health officials and vaccine developers last week -- holds up.
However, both trials are still taking place and the final numbers could change.
Moderna's vaccine appears to be easier to store as it remains stable at minus 20C for up to six months and can be kept in a standard fridge for up to a month.
Pfizer's vaccine needs ultra-cold storage at around minus 75C, but it can be kept in the fridge for five days.